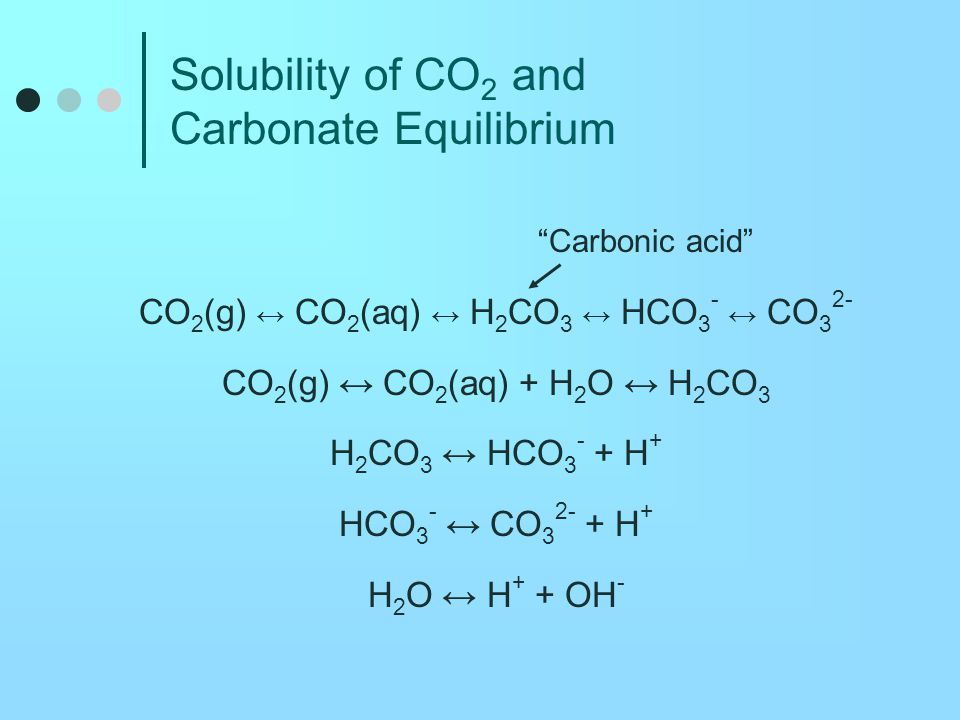
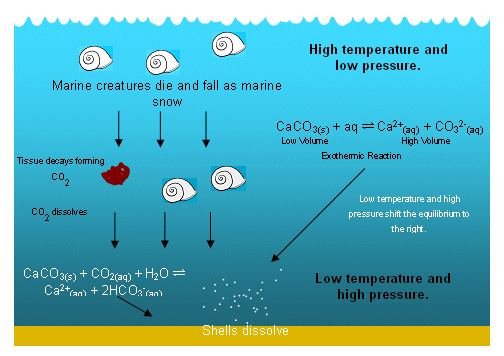
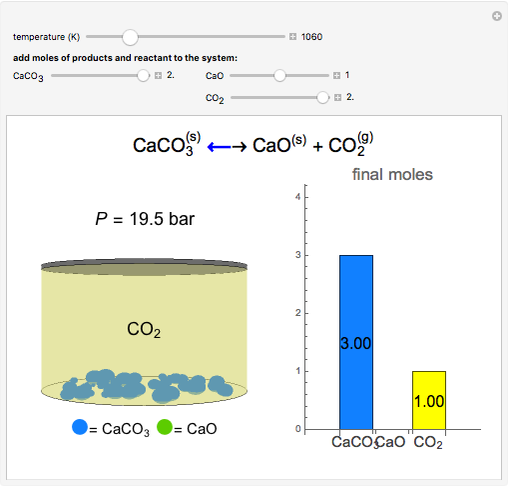
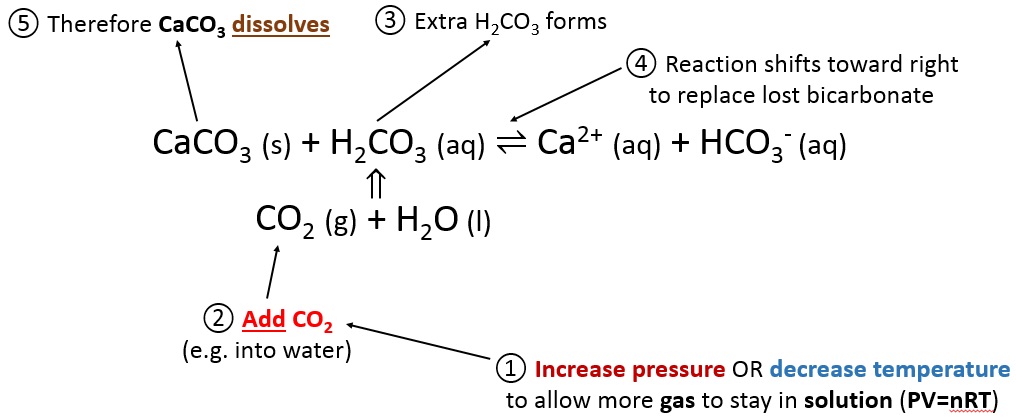
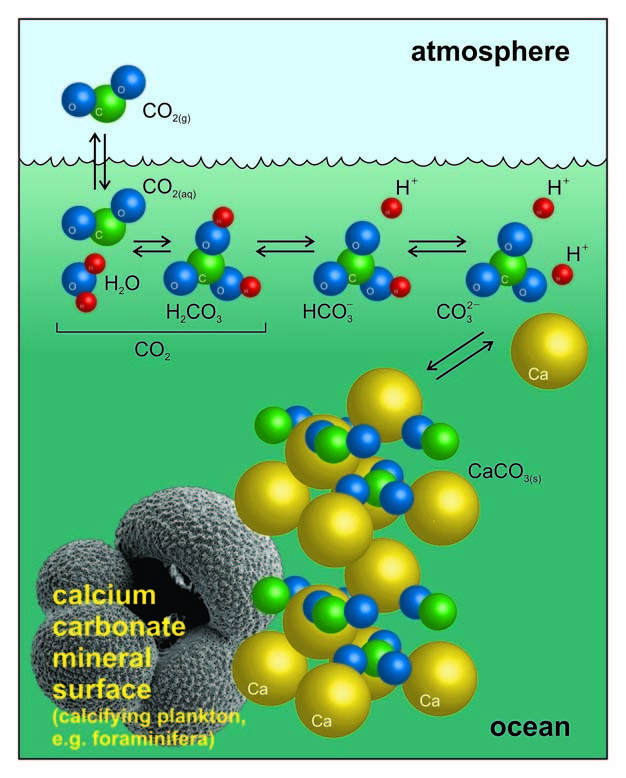
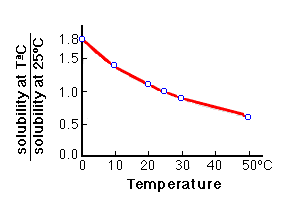
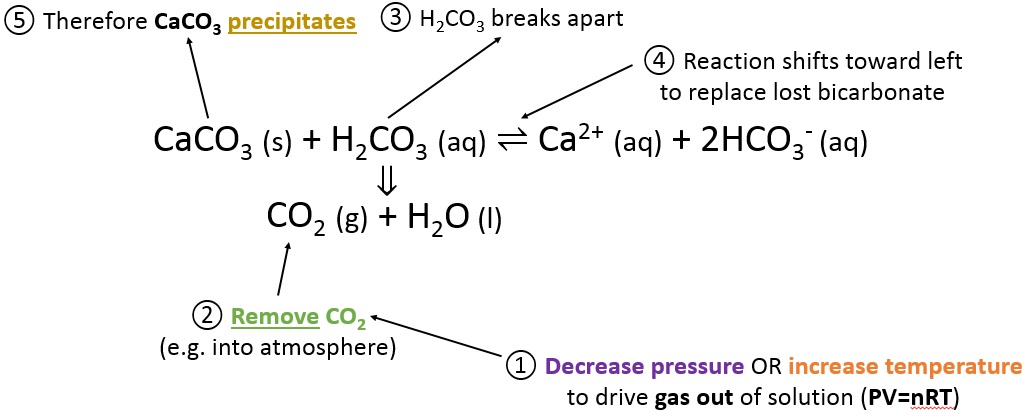
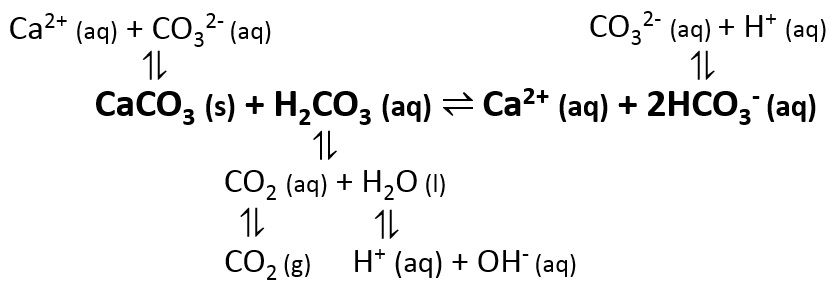Summary of the reactions between carbon dioxide (CO2) with water (H2O)... | Download Scientific Diagram
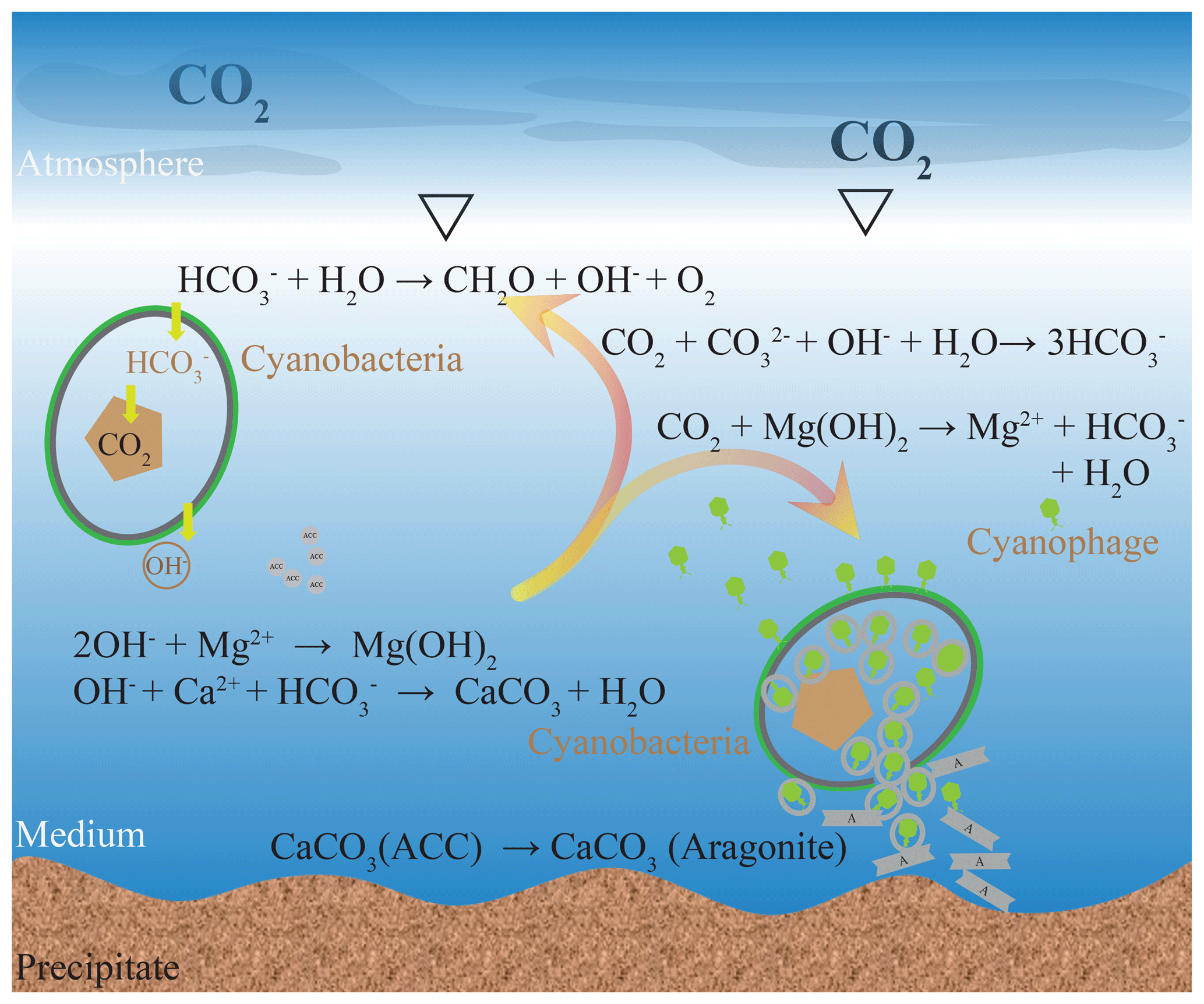
BG - Precipitation of calcium carbonate mineral induced by viral lysis of cyanobacteria: evidence from laboratory experiments
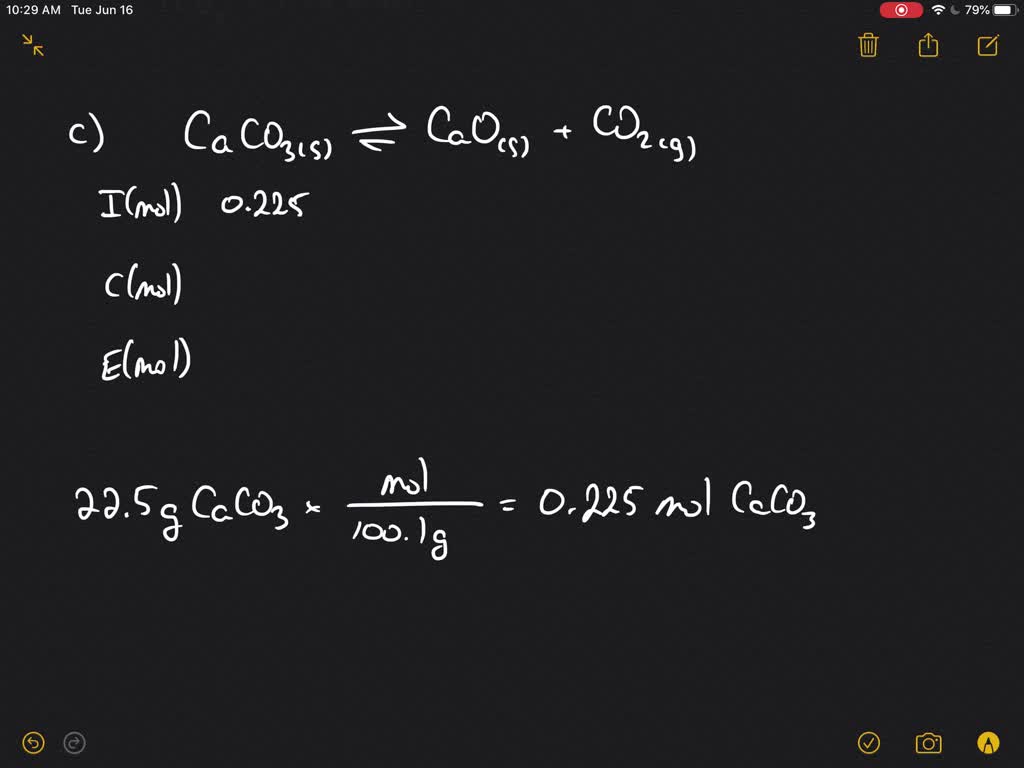
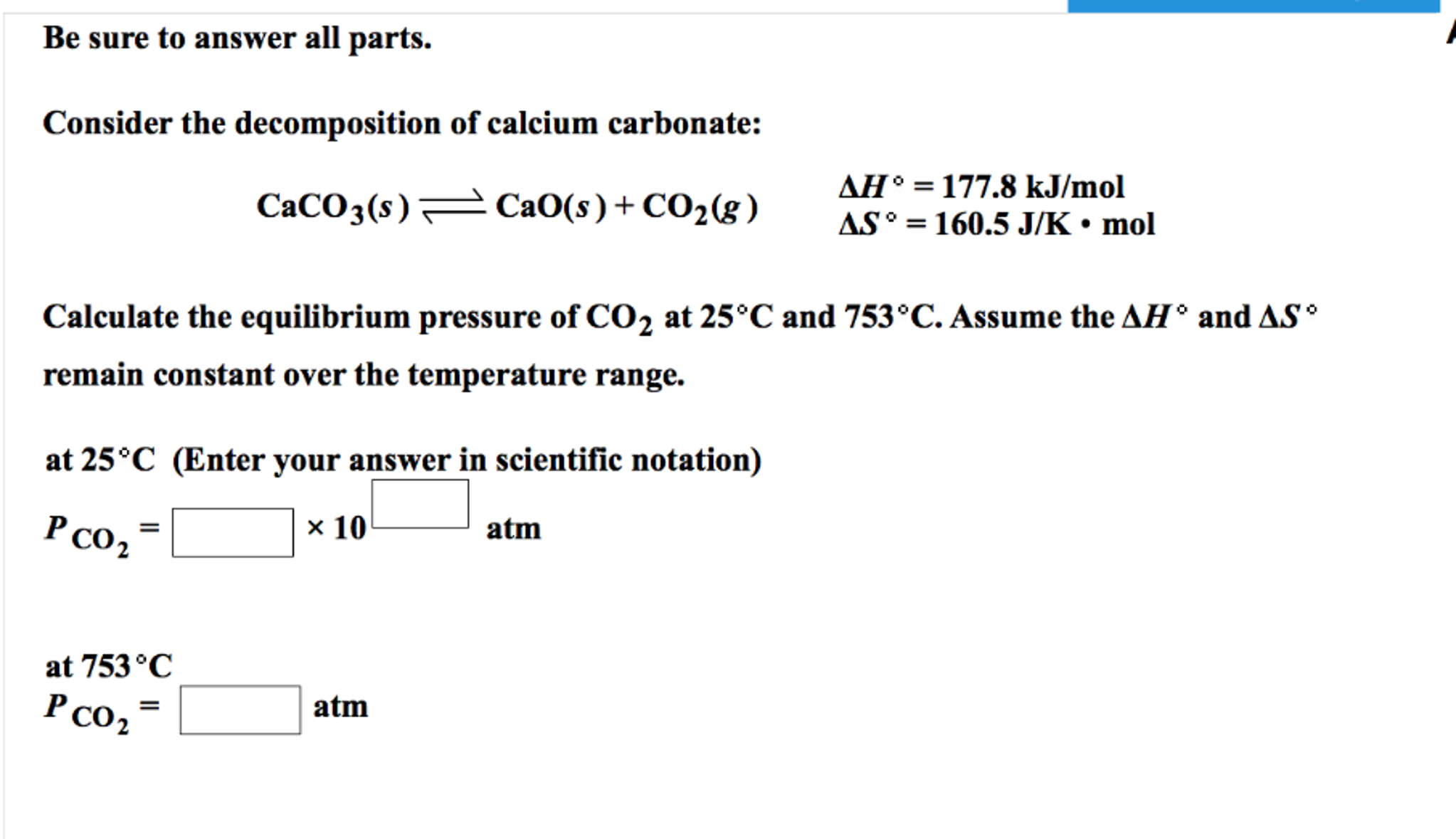
SOLVED:A The dissociation of calcium carbonate has an equilibrium constant of Kp=1.16 at 800^∘ C CaCO3(s) ⇄CaO(s)+CO2(g) (a) What is Kc for the reaction? (b) If you place 22.5 g of CaCO3

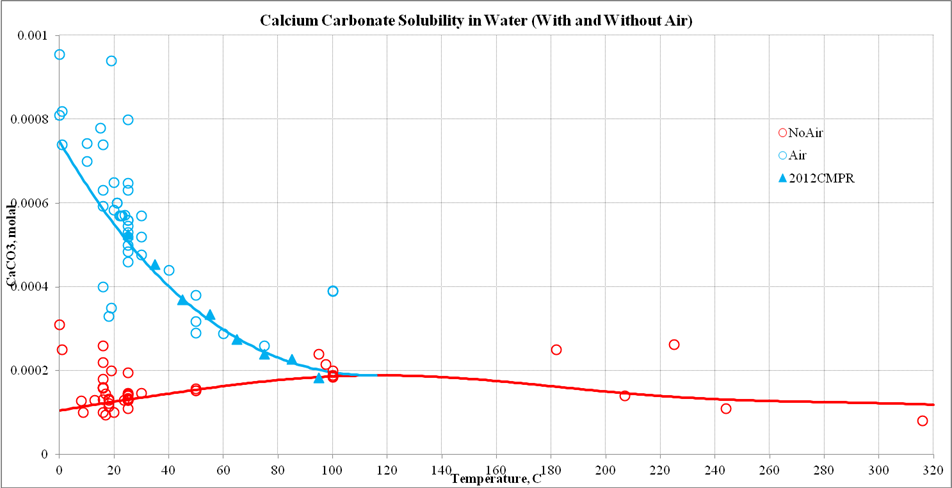
Calcium carbonate equilibrium or saturation (SI = 0, at 25 ◦ C and {HCO... | Download Scientific Diagram
Thermodynamic Simulation of Carbonate Cements-Water-Carbon Dioxide Equilibrium in Sandstone for Prediction of Precipitation/Dissolution of Carbonate Cements | PLOS ONE
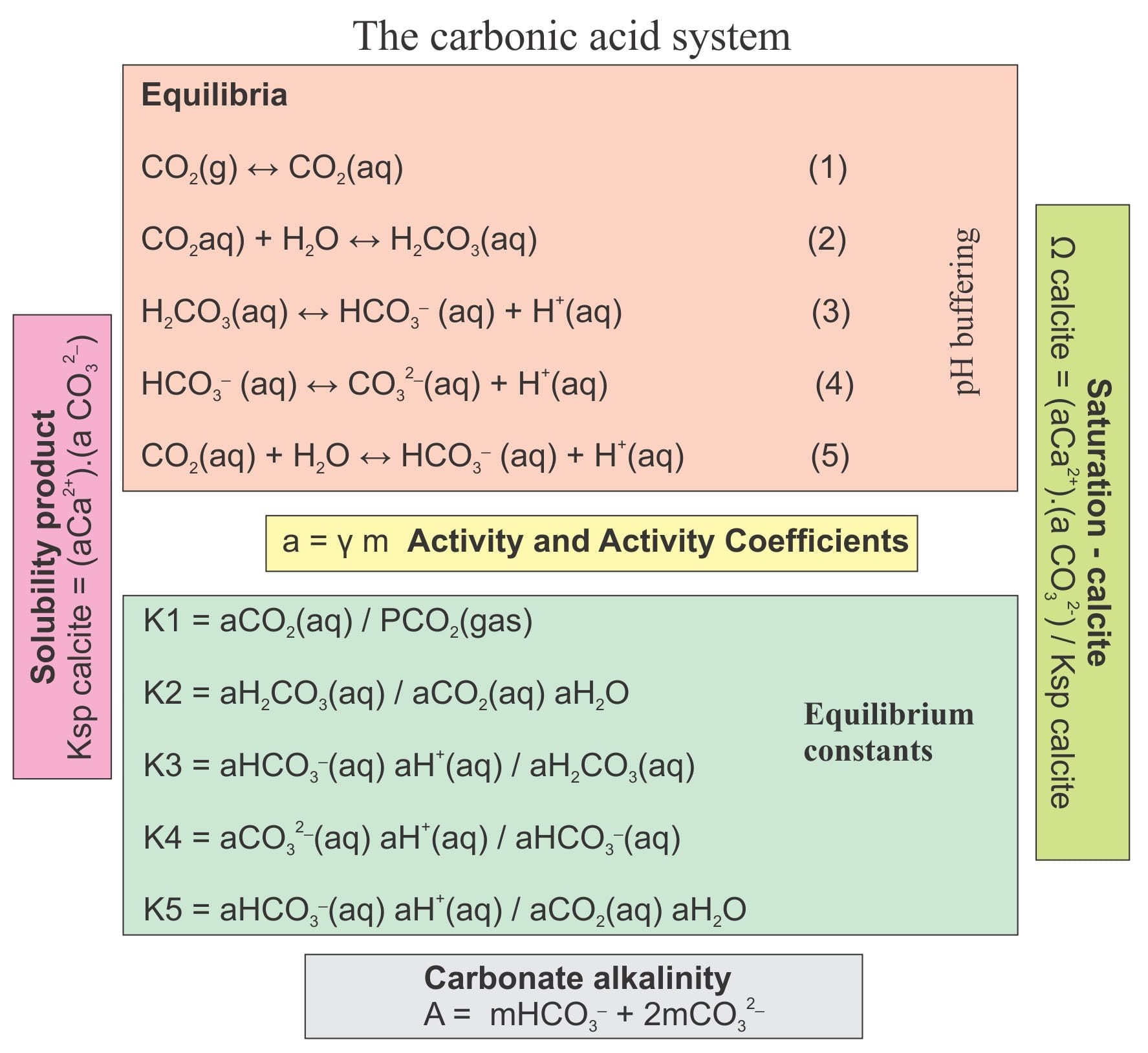
![Equilibriums and equilibrium constant values for carbonate systems [31] | Download Scientific Diagram Equilibriums and equilibrium constant values for carbonate systems [31] | Download Scientific Diagram](https://www.researchgate.net/publication/331530017/figure/tbl7/AS:733087658962947@1551793126689/Equilibriums-and-equilibrium-constant-values-for-carbonate-systems-31.png)
Equilibriums and equilibrium constant values for carbonate systems [31] | Download Scientific Diagram
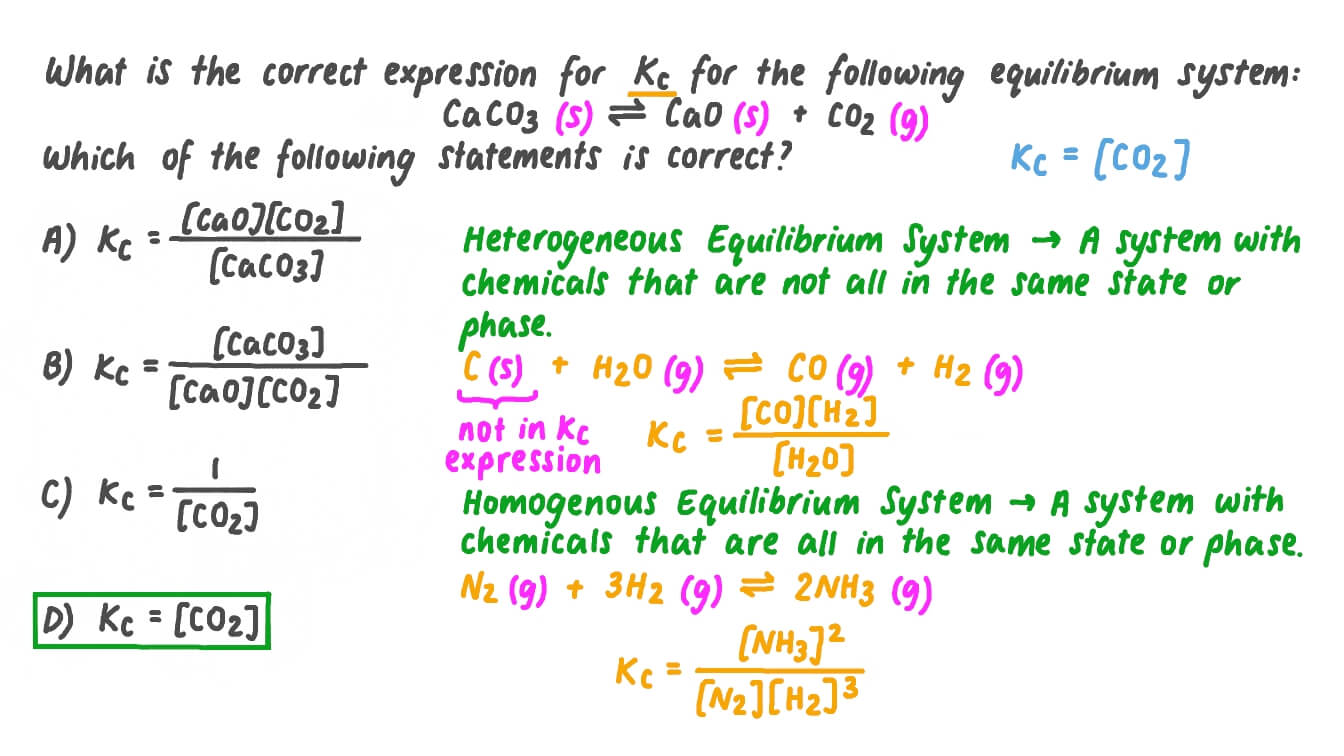
Question Video: Determining the Expression for the Equilibrium Constant for the Decomposition of Calcium Carbonate | Nagwa